• The United Nations (UN) officially declared 2019 as the International Year of the Periodic Table of Chemical Elements (IYPT2019).
• This coincides with the 150th anniversary of Russian chemist Dmitri Mendeleev’s pioneering version of the Periodic Table.
• Initially consisting of 63 known chemical elements, Mendeleev’s version served as the foundation for the Periodic Table as we know it.
Some love it, some fear it… and many, as students, had to memorize it.
Depending on who you ask, the Periodic Table of Elements is either an invaluable tool or an elaborate torture device. Kidding aside, its significance to chemistry — and to science as a whole — simply can’t be denied. It is perhaps the most important chemistry reference in existence, successfully fitting so much information into such a compact, well-organized system. While it may initially look daunting, it’s actually quite easy to use, if you know what you’re looking at.

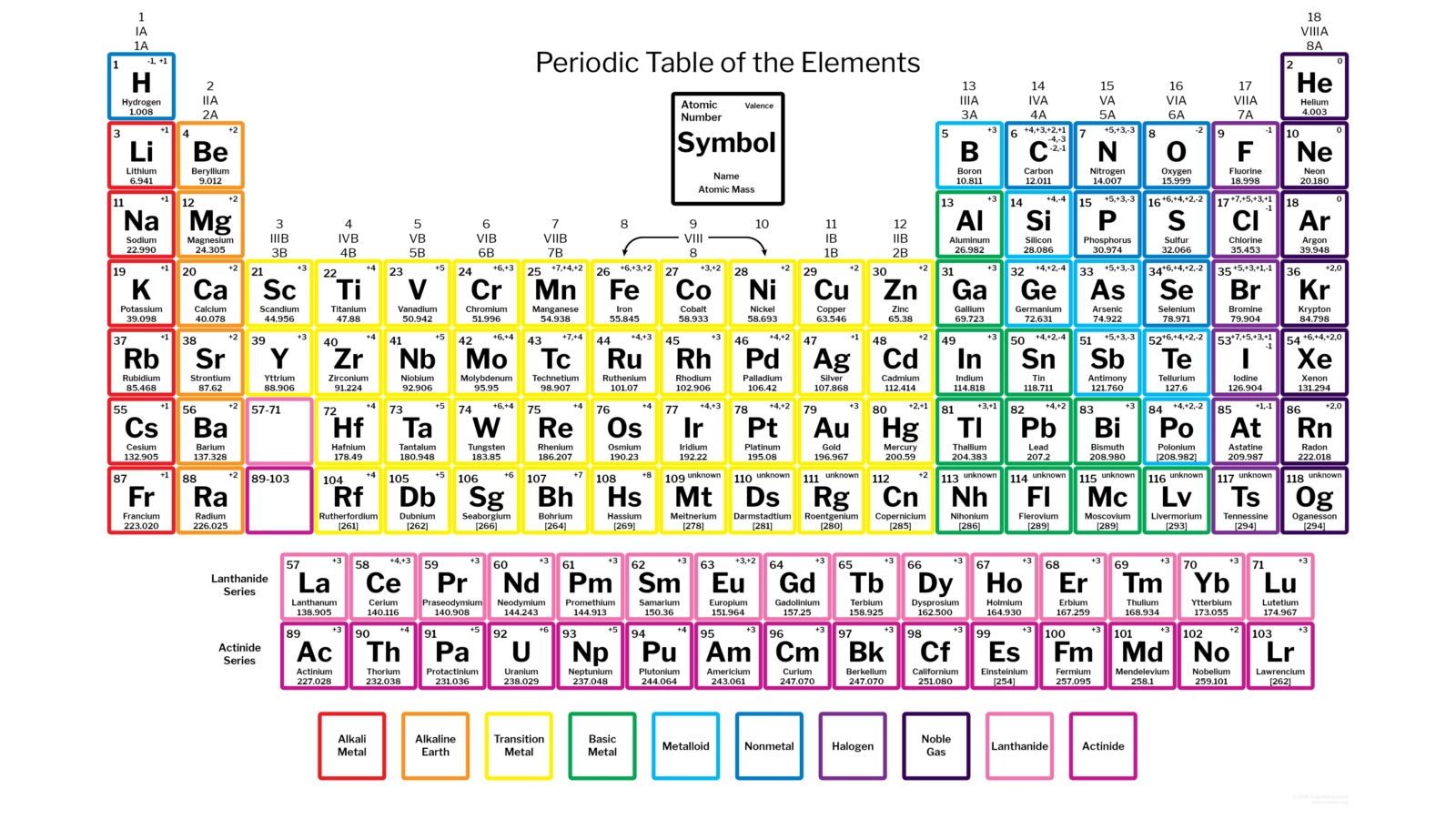
Take hydrogen, for example. It’s the first element on the table (we’ll talk about the “why” later), and is located on the upper leftmost square. Each square on the Periodic Table contains the element’s atomic symbol (one or two letters), atomic number (the number of protons in an element), and atomic weight or atomic mass (the sum of the atom’s protons and neutrons). Pretty straightforward, right?
On December 20, 2017, the United Nations (UN) General Assembly declared 2019 as the International Year of the Periodic Table of Chemical Elements (IYPT 2019). The decision serves a twofold purpose: to emphasize the significance and applications of the Periodic Table to society on an international scale, and to celebrate the 150th year since it was first published.
With that in mind, let’s take a look at the story of how the Periodic Table came to be — a story that mostly revolves around a stubborn man and his deck of cards.
Ordered chaos
Chemists actually started identifying chemical elements (“pure” substances with just one atom) in the 1700s. In 1789, Antoine Lavoisier published Elements of Chemistry, in which he described 33 elements (includings some strange ones, like “light” and “caloric”). The problem back then was that a standard, universal system of representing these elements didn’t exist. As you can probably imagine, this made things quite complicated.
In 1860, European chemists gathered at the Karlsruhe Congress — said to be the first international chemistry conference — to sort this out. To simplify things, they assigned an atomic weight of 1 to hydrogen, the lightest element. Hydrogen would then serve as the basis of comparison for the weights of all other elements. An element ten times heavier than hydrogen, for instance, would have an atomic weight of 10.
The establishment of a measurement system for the elements prompted scientists to devise a standardized method of grouping them. The first was French mining engineer Alexandre-Émile Béguyer de Chancourtois’ telluric screw (a list of the elements, arranged by increasing atomic weight, on graphing paper wrapped around a cylinder) in 1862. Two years later, British chemist John Newlands published his Law of Octaves. He based it on his observation that every eighth element seemed to repeat certain properties. While his proposed system was largely ridiculed and ultimately discarded, he was on the right track (somewhat).
Enter a Russian chemist named Dmitri Mendeleev, who was determined to organize the known elements (63 at the time) in a manner that mostly made sense.

Elementary, my dear textbook
Mendeleev’s quest began when he was writing Osnovy Khimii (Principles of Chemistry), a textbook for the course he was teaching. Eventually, he came to a similar realization as Newlands, albeit independently and in a different manner.
Mendeleev started with hydrogen, oxygen, nitrogen, and carbon — the four “standard” elements — and then proceeded to arrange the rest. However, this was not enough to develop a well-organized system. It’s worth noting that at the time, chemists were exclusively grouping elements based on either their atomic weight or shared properties, and not both. (Mendeleev would later incorporate both methods of categorization into a single system.)
While Newlands’ discovery was influenced by musical scales, Mendeleev’s was supposedly a product of a three-day solitaire marathon.
Mendeleev allegedly prepared a special set of 63 cards, with each card containing its designated element’s atomic weight and properties. In February 1869, after three days of arranging and rearranging his cards, he noticed something: that there were gaps in the order of the elements’ atomic mass. (Oddly enough, Mendeleev said that this idea came to him in a dream.)
Ultimately, Mendeleev realized that certain elemental properties recurred at specific intervals — in other words, a periodicity — which allowed him to group similar elements vertically.

Period(ic) piece
Mendeleev’s proposed system arranged the elements in rows called periods, while simultaneously lining up elements with similar properties in vertical columns called families. This system wasn’t just easy to read; it was also easy to interpret. A single glance could tell you how quickly a certain element would react or bond with others, its melting and boiling point, and the elements with which it shares specific characteristics.
But what about the gaps? Simple: Mendeleev treated them as elements that weren’t discovered yet, and allocated spaces for them.
He took it a step further by predicting these unknown elements’ atomic masses and chemical properties. When three of these elements — the ones now known as gallium, germanium, and scandium — were discovered in subsequent years, Mendeleev was proven right. This turned out to be the deciding factor in the scientific community’s acceptance of Mendeleev’s Periodic Table.
Mendeleev didn’t stop there, though. An updated version of his Periodic Table, published in 1871, explicitly demonstrated the formulas for oxides by including an oxide ratio column.

Nowadays, we arrange the elements in each row following Mendeleev’s columns (i.e. the groups are now displayed vertically instead of horizontally). Furthermore, the number of known elements has almost doubled. From the initial 63 elements, there are now 118: 94 elements that naturally occur on Earth, and 24 synthetic elements.
Crediting chemists
To attribute the Periodic Table solely to Mendeleev would do other chemists a tremendous disservice, though. After all, Mendeleev based his system on the findings of chemists before him who attempted to figure out this chemical conundrum.
Interestingly, a German chemist named Julius Lothar Meyer devised a table similar to Mendeleev’s in 1870. Meyer and Mendeleev shared many striking similarities. They were both attendees of the Karlsruhe Congress, they were both teachers, and they both studied under the chemist Robert Bunsen. When it came to the gaps, however, where Meyer zigged, Mendeleev zagged. The former avoided predicting new elements because he felt it wasn’t his job, while the latter embraced it. Ironically, Mendeleev’s willingness to fill in the gaps was a direct result of his stubborn refusal to accept what we now regard as scientific truths: the existence of the atom, the concept of radioactivity, and even the discovery of the electron.
Still, Mendeleev published his findings first. Thus, it was his Periodic Table that served as the framework for modern versions, and not Meyer’s.
With IYPT2019, the UN hopes to emphasize science and chemistry’s significance over the last 150 years, up to today. Within the story of the Periodic Table’s genesis, however, lies another important lesson: When commitment and curiosity come together, a certain kind of chemistry can come out of it.
Cover photo: Wikimedia Commons
References
- http://blogs.springeropen.com/springeropen/2016/02/10/mendeleev-and-the-periodic-table/
- https://corrosion-doctors.org/Periodic/Periodic-Mendeleev.htm
- https://iupac.org/united-nations-proclaims-international-year-periodic-table-chemical-elements/
- https://www.euchems.eu/iypt2019/about/
- https://www.khanacademy.org/partner-content/big-history-project/stars-and-elements/knowing-stars-elements/a/dmitri-mendeleev
- https://www.sciencehistory.org/distillations/magazine/an-element-of-order
Author: Mikael Angelo Francisco
Bitten by the science writing bug, Mikael has years of writing and editorial experience under his belt. As the editor-in-chief of FlipScience, Mikael has sworn to help make science more fun and interesting for geeky readers and casual audiences alike.